Multimedia
In vitro motility assay
(Video 1 and 2)
Consists in observing fluorescently labeled actin filaments as they get propelled by myosin molecules adhered randomly to a nitrocellulose-coated coverslip. The velocity of actin filament propulsion is determined in presence of MgATP. (TRITC-labeled skeletal muscle actin filaments propelled by smooth muscle myosin).
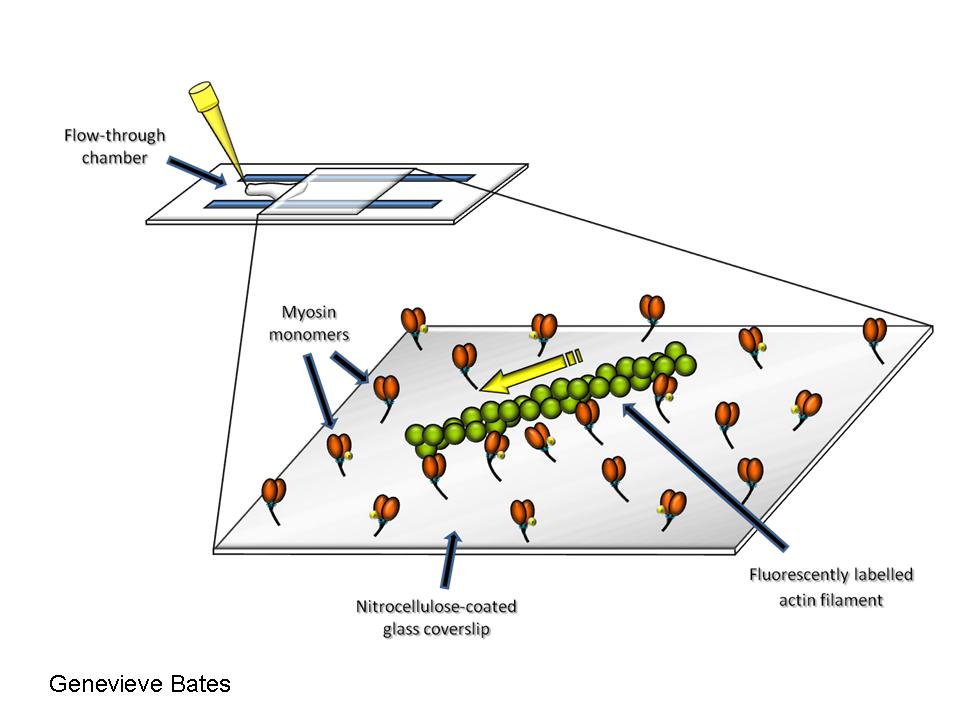
Video 1
Video 2
TRITC-labeled skeletal muscle actin filaments decorated with tropomyosin-alpha and beta and propelled by smooth muscle myosin.
alpha-actinin force assay
(Video 3a, 3b, 3c)
alpha-actinin force assay: This assay uses a-actinin to attach actin to the nitrocellulose on the coverslip and retard the propulsion by myosin. The greater the concentration of a-actinin needed to stop the movement of actin, the stronger the force generated by the actomyosin complex. a) Low [alpha-actinin]: normal actin movement; b) Average [alpha-actinin]: slower actin movement; c) High [a-actinin]: no actin movement.
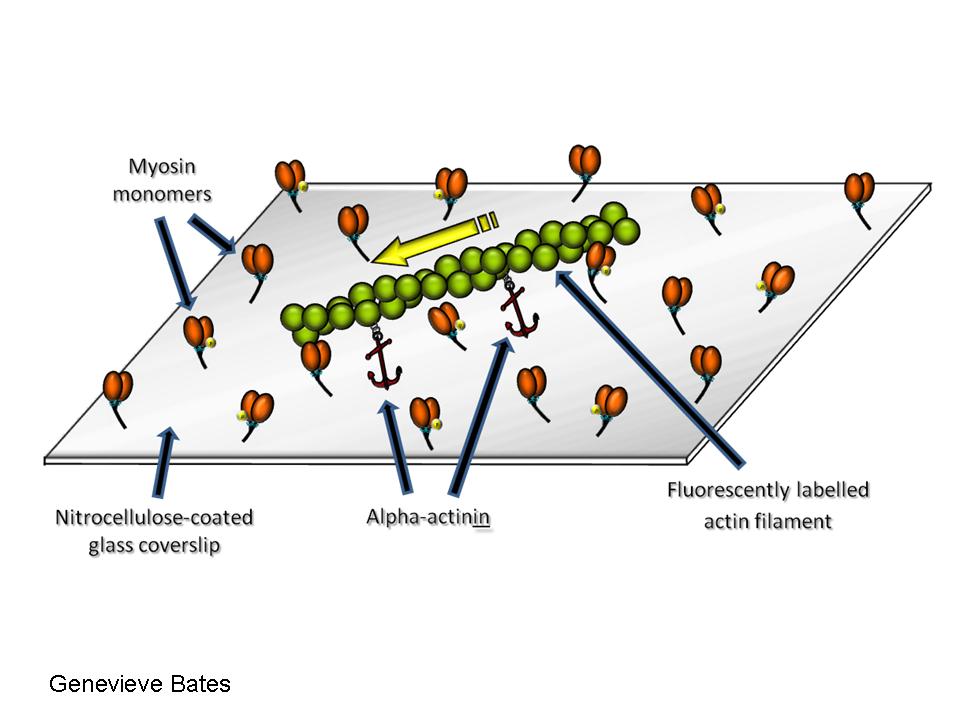
Video 3.a
Video 3.b
Video 3.c
Laser trap unbinding assay
Video 4: Laser trap unbinding assay: A single beam laser trap is used to capture a 3µm diameter bead coated with NEM-myosin (small bead in video). A TRITC-labeled actin filament is attached to the bead and brought in contact with myosin randomly adhered to a 4.5µm pedestal (large bead in video) on a coverslip. After allowing time for binding of myosin to actin, the pedestal is moved away from the trap at constant velocity. Initially, the trapped bead follows the pedestal, offset from the center of the trap. When the pulling force exerted by the trap exceeds the binding force of the myosin molecules, the microsphere springs back into the trap center, its unloaded position. The force of unbinding is equal to the product of the maximal distance between the bead and the trap center by the trap stiffness.
Video 4



